|
Themenwebsites
Medizin A - Z
im Info-Netzwerk
Medizin 2000
2.1.2026
|
|
Impfschema
für Erwachsene - Jahr 2010
Recommended
Adult Immunization Schedule
United States, 2010
The Advisory Committee on Immunization Practices (ACIP) annually
reviews the recommended Adult Immunization Schedule to ensure
that the schedule reflects current recommendations for the licensed
vaccines. In October 2009, ACIP approved the Adult Immunization
Schedule for 2010, which includes several changes. A bivalent
human papillomavirus vaccine (HPV2) was licensed for use in
females in October 2009. ACIP recommends vaccination of females
with either HPV2 or the quadrivalent human papillomavirus vaccine
(HPV4). HPV4 was licensed for use in males in October 2009,
and ACIP issued a permissive recommendation for use in males.
Introductory sentences were added to the footnotes for measles,
mumps, rubella, influenza, pneumococcal, hepatitis A, hepatitis
B, and meningococcal vaccines. Clarifications were made to the
footnotes for measles, mumps, rubella, influenza, hepatitis
A, meningococcal, and Haemophilus influenza type
b vaccines, and schedule information was added to the hepatitis
B vaccine footnote.
Additional information is available
as follows: schedule (in English and Spanish)
at
http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm;
adult vaccination at http://www.cdc.gov/vaccines/default.htm;
ACIP statements for specific
vaccines at http://www.cdc.gov/vaccine/pubs/acip-list.htm;
and reporting adverse events at http://www.vaers.hhs.gov or
by telephone, or
by telephone,
800-822-7967.
Changes
for 2010
Footnotes (Figures 1 and 2)
-
The
human papillomavirus (HPV) footnote (#2)
includes language that a bivalent HPV vaccine (HPV2) has
been licensed for use in females. Either HPV2 or the quadrivalent
human papillomavirus vaccine (HPV4) can be used for vaccination
of females aged 19 through 26 years. In addition, language
has been added to indicate that ACIP issued a permissive
recommendation for use of HPV4 in males.
-
The
measles, mumps, rubella (MMR) footnote
(#5) has language added to clarify which adults born during
or after 1957 do not need 1 or more doses of MMR vaccine
for the measles and mumps components, and clarifies which
women should receive a dose of MMR vaccine. Also, interval
dosing information has been added to indicate when a second
dose of MMR vaccine should be administered. Language has
been added to highlight recommendations for vaccinating
health-care personnel born before 1957 routinely and during
outbreaks.
-
The
term “seasonal” has been added
to the influenza footnote (#6).
-
The
hepatitis A footnote (#9) has language
added to indicate that unvaccinated persons who anticipate
close contact with an international adoptee should consider
vaccination.
-
The
hepatitis B footnote (#10) has language
added to include schedule information for the 3-dose hepatitis
B vaccine.
-
The
meningococcal vaccine footnote (#11) clarifies
which vaccine formulations are preferred for adults aged
≤55 years and ≥56 years, and which vaccine formulation
can be used for revaccination. New examples have been added
to demonstrate who should and should not be considered for
revaccination.
-
The selected conditions for Haemophilus influenza type
b (Hib) footnote (#13) clarifies which high-risk
persons may receive 1 dose of Hib vaccine.
The Recommended Adult Immunization Schedule has been approved
by the Advisory Committee on Immunization Practices, the American
Academy of Family Physicians, the American College of Obstetricians
and Gynecologists, and the American College of Physicians.
Suggested citation: Centers for Disease Control and
Prevention. Recommended adult immunization schedule---United
States, 2010. MMWR 2010;59(1).
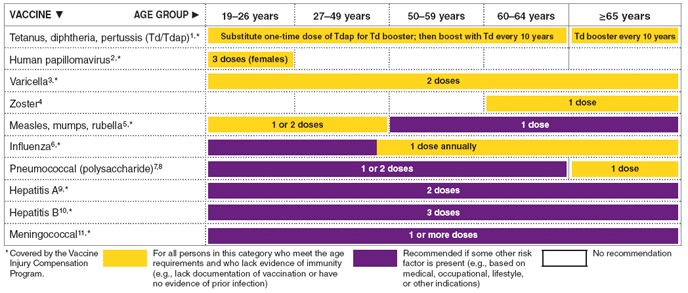
FIGURE
1. Recommended adult immunization schedule, by vaccine
and age group - United States, 2010
The
figure above shows the recommended adult immunization schedule,
by vaccine and age group for the United States in 2010.
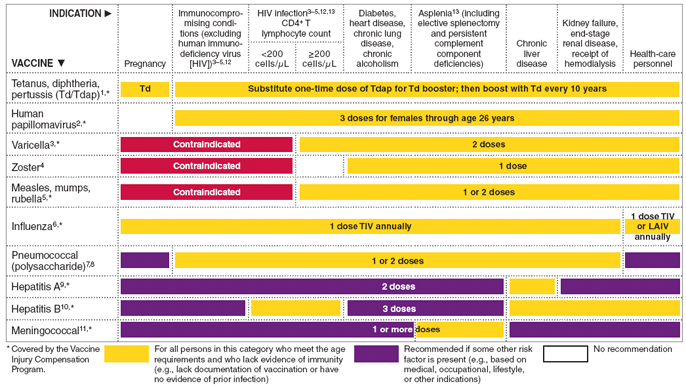
FIGURE
2. Vaccines that might be indicated for adults, based
on medical and other indications - United States, 2010
The
figure above shows vaccines that might be indicated for adults,
based on medical and other indications in the United States
for 2010.
-
Tetanus, diphtheria, and acellular pertussis (Td/Tdap)
vaccination
Tdap should replace a single dose of Td for adults aged
19--64 years who have not received a dose of Tdap previously.
Adults with uncertain or incomplete history of primary vaccination
series with tetanus and diphtheria toxoid-containing vaccines
should begin or complete a primary vaccination series. A
primary series for adults is 3 doses of tetanus and diphtheria
toxoid-containing vaccines; administer the first 2 doses
at least 4 weeks apart and the third dose 6--12 months after
the second; Tdap can substitute for any one of the doses
of Td in the 3-dose primary series. The booster dose of
tetanus and diphtheria toxoid-containing vaccine should
be administered to adults who have completed a primary series
and if the last vaccination was received >10 years
previously. Tdap or Td vaccine may be used, as indicated.
If a woman is pregnant and received the last Td vaccination >10
years previously, administer Td during the second or third
trimester. If the woman received the last Td vaccination
<10 years previously, administer Tdap during the immediate
postpartum period. A dose of Tdap is recommended for postpartum
women, close contacts of infants aged <12 months, and
all health-care personnel with direct patient contact if
they have not previously received Tdap. An interval as short
as 2 years from the last Td vaccination is suggested; shorter
intervals can be used. Td may be deferred during pregnancy
and Tdap substituted in the immediate postpartum period,
or Tdap can be administered instead of Td to a pregnant
woman.
Consult the ACIP statement for recommendations for giving
Td as prophylaxis in wound management.
-
Human papillomavirus (HPV) vaccination
HPV vaccination is recommended at age 11 or 12 years with
catch-up vaccination at ages 13 through 26 years.
Ideally, vaccine should be administered before potential
exposure to HPV through sexual activity; however, females
who are sexually active should still be vaccinated consistent
with age-based recommendations. Sexually active females
who have not been infected with any of the four HPV vaccine
types (types 6, 11, 16, 18, all of which HPV4 prevents)
or any of the two HPV vaccine types (types 16 and 18, both
of which HPV2 prevents) receive the full benefit of the
vaccination. Vaccination is less beneficial for females
who have already been infected with one or more of the HPV
vaccine types. HPV4 or HPV2 can be administered to persons
with a history of genital warts, abnormal Papanicolaou test,
or positive HPV DNA test, because these conditions are not
evidence of prior infection with all vaccine HPV types.
HPV4 may be administered to males aged 9 through 26 years
to reduce their likelihood of acquiring genital warts. HPV4
would be most effective when administered before exposure
to HPV through sexual contact.
A complete series for either HPV4 or HPV2 consists of 3
doses. The second dose should be administered 1--2 months
after the first dose; the third dose should be administered
6 months after the first dose.
Although HPV vaccination is not specifically recommended
for persons with the medical indications described in Figure
2, "Vaccines that might be indicated for adults based
on medical and other indications," it may be administered
to these persons because the HPV vaccine is not a live-virus
vaccine. However, the immune response and vaccine efficacy
might be less for persons with the medical indications described
in Figure 2 than in persons who do not have the medical
indications described or who are immunocompetent. Health-care
personnel are not at increased risk because of occupational
exposure and should be vaccinated consistent with age-based
recommendations.
-
Varicella vaccination
All adults without evidence of immunity to varicella should
receive 2 doses of single-antigen varicella vaccine if not
previously vaccinated or the second dose if they have received
only 1 dose, unless they have a medical contraindication.
Special consideration should be given to those who 1) have
close contact with persons at high risk for severe disease
(e.g., health-care personnel and family contacts of persons
with immunocompromising conditions) or 2) are at high risk
for exposure or transmission (e.g., teachers; child-care
employees; residents and staff members of institutional
settings, including correctional institutions; college students;
military personnel; adolescents and adults living in households
with children; nonpregnant women of childbearing age; and
international travelers).
Evidence of immunity to varicella in adults includes any
of the following: 1) documentation of 2 doses of varicella
vaccine at least 4 weeks apart; 2) U.S.-born before 1980
(although for health-care personnel and pregnant women,
birth before 1980 should not be considered evidence of immunity);
3) history of varicella based on diagnosis or verification
of varicella by a health-care provider (for a patient reporting
a history of or having an atypical case, a mild case, or
both, health-care providers should seek either an epidemiologic
link with a typical varicella case or to a laboratory-confirmed
case or evidence of laboratory confirmation, if it was performed
at the time of acute disease); 4) history of herpes zoster
based on diagnosis or verification of herpes zoster by a
health-care provider; or 5) laboratory evidence of immunity
or laboratory confirmation of disease.
Pregnant women should be assessed for evidence of varicella
immunity. Women who do not have evidence of immunity should
receive the first dose of varicella vaccine upon completion
or termination of pregnancy and before discharge from the
health-care facility. The second dose should be administered
4--8 weeks after the first dose.
-
Herpes zoster vaccination
A single dose of zoster vaccine is recommended for adults
aged >60 years regardless of whether they report
a prior episode of herpes zoster. Persons with chronic medical
conditions may be vaccinated unless their condition constitutes
a contraindication.
-
Measles, mumps, rubella (MMR) vaccination
Adults born before 1957 generally are considered immune
to measles and mumps.
Measles component: Adults born during
or after 1957 should receive 1 or more doses of MMR vaccine
unless they have 1) a medical contraindication; 2) documentation
of vaccination with 1 or more doses of MMR vaccine; 3) laboratory
evidence of immunity; or 4) documentation of physician-diagnosed
measles.
A second dose of MMR vaccine, administered 4 weeks after
the first dose, is recommended for adults who 1) have been
recently exposed to measles or are in an outbreak setting;
2) have been vaccinated previously with killed measles vaccine;
3) have been vaccinated with an unknown type of measles
vaccine during 1963--1967; 4) are students in postsecondary
educational institutions; 5) work in a health-care facility;
or 6) plan to travel internationally.
Mumps component: Adults born during
or after 1957 should receive 1 dose of MMR vaccine unless
they have 1) a medical contraindication; 2) documentation
of vaccination with 1 or more doses of MMR vaccine; 3) laboratory
evidence of immunity; or 4) documentation of physician-diagnosed
mumps.
A second dose of MMR vaccine, administered 4 weeks after
the first dose, is recommended for adults who 1) live in
a community experiencing a mumps outbreak and are in an
affected age group; 2) are students in postsecondary educational
institutions; 3) work in a health-care facility; or 4) plan
to travel internationally.
Rubella component: 1 dose of MMR vaccine
is recommended for women who do not have documentation of
rubella vaccination, or who lack laboratory evidence of
immunity. For women of childbearing age, regardless of birth
year, rubella immunity should be determined, and women should
be counseled regarding congenital rubella syndrome. Women
who do not have evidence of immunity should receive MMR
vaccine upon completion or termination of pregnancy and
before discharge from the health-care facility.
Health-care personnel born before 1957: For unvaccinated
health-care personnel born before 1957 who lack laboratory
evidence of measles, mumps, and/or rubella immunity or laboratory
confirmation of disease, health-care facilities should consider
vaccinating personnel with 2 doses of MMR vaccine at the
appropriate interval (for measles and mumps) and 1 dose
of MMR vaccine (for rubella), respectively.
During outbreaks, health-care facilities should recommend
that unvaccinated health-care personnel born before 1957,
who lack laboratory evidence of measles, mumps, and/or rubella
immunity or laboratory confirmation of disease, receive
2 doses of MMR vaccine during an outbreak of measles or
mumps, and 1 dose during an outbreak of rubella.
Complete information about evidence of immunity is available
at http://www.cdc.gov/vaccines/recs/provisional/default.htm.
-
Seasonal influenza vaccination
Vaccinate all persons aged >50 years and any younger
persons who would like to decrease their risk for influenza.
Vaccinate persons aged 19 through 49 years with any of the
following indications.
Medical: Chronic disorders of the
cardiovascular or pulmonary systems, including asthma; chronic
metabolic diseases (including diabetes mellitus); renal
or hepatic dysfunction, hemoglobinopathies, or immunocompromising
conditions (including immunocompromising conditions caused
by medications or HIV); cognitive, neurologic, or neuromuscular
disorders; and pregnancy during the influenza season. No
data exist on the risk for severe or complicated influenza
disease among persons with asplenia; however, influenza
is a risk factor for secondary bacterial infections that
can cause severe disease among persons with asplenia.
Occupational: All health-care personnel,
including those employed by long-term care and assisted-living
facilities, and caregivers of children aged <5 years.
Other: Residents of nursing homes
and other long-term care and assisted-living facilities;
persons likely to transmit influenza to persons at high
risk (e.g., in-home household contacts and caregivers of
children aged <5 years, persons aged >50 years,
and persons of all ages with high-risk conditions).
Healthy, nonpregnant adults aged <50 years without high-risk
medical conditions who are not contacts of severely immunocompromised
persons in special-care units may receive either intranasally
administered live, attenuated influenza vaccine (FluMist)
or inactivated vaccine. Other persons should receive the
inactivated vaccine.
-
Pneumococcal polysaccharide (PPSV) vaccination
Vaccinate all persons with the following indications.
Medical: Chronic lung disease (including asthma); chronic
cardiovascular diseases; diabetes mellitus; chronic liver
diseases, cirrhosis; chronic alcoholism; functional or anatomic
asplenia (e.g., sickle cell disease or splenectomy [if elective
spletnectomy is planned, vaccinate at least 2 weeks before
surgery]); immunocompromising conditions (including chronic
renal failure or nephrotic syndrome); and cochlear implants
and cerebrospinal fluid leaks. Vaccinate as close to HIV
diagnosis as possible.
Other: Residents of nursing homes or long-term care
facilities and persons who smoke cigarettes. Routine use
of PPSV is not recommended for American Indians/Alaska Natives
or persons aged <65 years unless they have underlying
medical conditions that are PPSV indications. However, public
health authorities may consider recommending PPSV for American
Indians/Alaska Natives and persons aged 50 through 64 years
who are living in areas where the risk for invasive pneumococcal
disease is increased.
-
Revaccination with PPSV
One-time revaccination after 5 years is recommended for
persons with chronic renal failure or nephrotic syndrome;
functional or anatomic asplenia (e.g., sickle cell disease
or splenectomy); and for persons with immunocompromising
conditions. For persons aged >65 years, one-time
revaccination is recommended if they were vaccinated >5
years previously and were aged <65 years at the time
of primary vaccination.
-
Hepatitis A vaccination
Vaccinate persons with any of the following indications
and any person seeking protection from hepatitis A virus
(HAV) infection.
Behavioral: Men who have sex with men and persons who
use injection drugs.
Occupational: Persons working with HAV-infected primates
or with HAV in a research laboratory setting.
Medical: Persons with chronic liver disease and persons
who receive clotting factor concentrates.
Other: Persons traveling to or working in countries
that have high or intermediate endemicity of hepatitis A
(a list of countries is available at
http://wwwn.cdc.gov/travel/contentdiseases.aspx).
Unvaccinated persons who anticipate close personal contact
(e.g., household contact or regular babysitting) with an
international adoptee from a country of high or intermediate
endemicity during the first 60 days after arrival of the
adoptee in the United States should consider vaccination.
The first dose of the 2-dose hepatitis A vaccine series
should be administered as soon as adoption is planned, ideally >2
weeks before the arrival of the adoptee.
Single-antigen vaccine formulations should be administered
in a 2-dose schedule at either 0 and 6--12 months (Havrix),
or 0 and 6--18 months (Vaqta). If the combined hepatitis
A and hepatitis B vaccine (Twinrix) is used, administer
3 doses at 0, 1, and 6 months; alternatively, a 4-dose schedule,
administered on days 0, 7, and 21--30 followed by a booster
dose at month 12 may be used.
-
Hepatitis B vaccination
Vaccinate persons with any of the following indications
and any person seeking protection from hepatitis B virus
(HBV) infection.
Behavioral: Sexually active persons who are not in
a long-term, mutually monogamous relationship (e.g., persons
with more than one sex partner during the previous 6 months);
persons seeking evaluation or treatment for a sexually transmitted
disease (STD); current or recent injection-drug users; and
men who have sex with men.
Occupational: Health-care personnel and public-safety
workers who are exposed to blood or other potentially infectious
body fluids.
Medical: Persons with end-stage renal disease, including
patients receiving hemodialysis; persons with HIV infection;
and persons with chronic liver disease.
Other: Household contacts and sex partners of persons
with chronic HBV infection; clients and staff members of
institutions for persons with developmental disabilities;
and international travelers to countries with high or intermediate
prevalence of chronic HBV infection (a list of countries
is available at http://wwwn.cdc.gov/travel/contentdiseases.aspx).
Hepatitis B vaccination is recommended for all adults in
the following settings: STD treatment facilities; HIV testing
and treatment facilities; facilities providing drug-abuse
treatment and prevention services; health-care settings
targeting services to injection-drug users or men who have
sex with men; correctional facilities; end-stage renal disease
programs and facilities for chronic hemodialysis patients;
and institutions and nonresidential day-care facilities
for persons with developmental disabilities.
Administer or complete a 3-dose series of hepatitis B vaccine
to those persons not previously vaccinated. The second dose
should be administered 1 month after the first dose; the
third dose should be administered at least 2 months after
the second dose (and at least 4 months after the first dose).
If the combined hepatitis A and hepatitis B vaccine (Twinrix)
is used, administer 3 doses at 0, 1, and 6 months; alternatively,
a 4-dose schedule, administered on days 0, 7, and 21--30
followed by a booster dose at month 12 may be used.
Adult patients receiving hemodialysis or with other immunocompromising
conditions should receive 1 dose of 40 µg/mL (Recombivax
HB) administered on a 3-dose schedule or 2 doses of 20 µg/mL
(Engerix-B) administered simultaneously on a 4-dose schedule
at 0, 1, 2, and 6 months.
-
Meningococcal vaccination
Meningococcal vaccine should be administered to persons
with the following indications.
Medical: Adults with anatomic or functional asplenia,
or persistent complement component deficiencies.
Other: First-year college students living in dormitories;
microbiologists routinely exposed to isolates of Neisseria
meningitidis; military recruits; and persons who travel
to or live in countries in which meningococcal disease is
hyperendemic or epidemic (e.g., the "meningitis belt"
of sub-Saharan Africa during the dry season [December through
June]), particularly if their contact with local populations
will be prolonged. Vaccination is required by the government
of Saudi Arabia for all travelers to Mecca during the annual
Hajj.
Meningococcal conjugate vaccine (MCV4) is preferred for
adults with any of the preceding indications who are aged
≤55 years; meningococcal polysaccharide vaccine (MPSV4)
is preferred for adults aged >56 years. Revaccination
with MCV4 after 5 years is recommended for adults previously
vaccinated with MCV4 or MPSV4 who remain at increased risk
for infection (e.g., adults with anatomic or functional
asplenia). Persons whose only risk factor is living in on-campus
housing are not recommended to receive an additional dose.
-
Immunocompromising conditions
Inactivated vaccines generally are acceptable (e.g., pneumococcal,
meningococcal, influenza [inactivated influenza vaccine])
and live vaccines generally are avoided in persons with
immune deficiencies or immunocompromising conditions. Information
on specific conditions is available at http://www.cdc.gov/vaccines/pubs/acip-list.htm.
-
Selected conditions for which Haemophilus
influenzae type
b (Hib) vaccine may be used
Hib vaccine generally is not recommended for persons aged >5
years. No efficacy data are available on which to base a
recommendation concerning use of Hib vaccine for older children
and adults. However, studies suggest good immunogenicity
in patients who have sickle cell disease, leukemia, or HIV
infection or who have had a splenectomy. Administering 1
dose of Hib vaccine to these high-risk persons who have
not previously received Hib vaccine is not contraindicated.
These
schedules indicate the recommended age groups and medical
indications for which administration of currently licensed
vaccines is commonly indicated for adults aged >19
years, as of January 1, 2009. Licensed combination vaccines
may be used whenever any components of the combination are
indicated and when the vaccine’s other components are
not contraindicated. For detailed recommendations on all vaccines,
including those that are used primarily for travelers or are
issued during the year, consult the manufacturers’ package
inserts and the complete statements from the Advisory Committee
on Immunization Practices (ACIP) (http://www.cdc.gov/vaccines/pubs/acip-list.htm).
Report all clinically significant postvaccination reactions
to the Vaccine Adverse Event Reporting System (VAERS). Reporting
forms and instructions on filing a VAERS report are available
at http://www.vaers.hhs.gov or
by telephone, 800-822-7967.
Information on how to file a Vaccine Injury Compensation Program
claim is available at http://www.hrsa.gov/vaccinecompensation or
by telephone, 800-338-2382. To file a claim for vaccine injury,
contact the U.S. Court of Federal Claims, 717 Madison Place,
N.W., Washington, D.C. 20005; telephone, 202-357-6400.
Additional information about the vaccines in this schedule,
extent of available data, and contraindications for vaccination
is available at http://www.cdc.gov/vaccines or
from the CDC-INFO Contact Center at 800-CDC-INFO (800-232-4636)
in English and Spanish, 24 hours a day, 7 days a week.
Use of trade names and commercial sources is for identification
only and does not imply endorsement by the U.S. Department
of Health and Human Services.
The recommendations in this schedule were approved by ACIP,
the American Academy of Family Physicians, the American College
of Obstetricians and Gynecologists, and the American College
of Physicians.
Department of Health and Human Services • Centers for
Disease Control and Prevention
|
|
Werbung im Info-Netzwerk Medizin 2000

Anzeige
Es gibt keine allgemein wissenschaftlich anerkannten Regeln welche Lebensmittel
in welcher Kombination, bzw. Menge gesund sind - und welche nicht.
Man kann den Eindruck gewinnen, dass jeder "Experte" eine individuell unterschiedliche Auffassung
von bestimmten Themen hat . Die wissenschaftlichen Erkenntnisse widersprechen einander und
die Ansichten variieren erheblich. Auf der Website
www.medizin-2000.de/gesunde-ernaehrung versuchen wir unsere Besucher durch unvoreingenommene Informationen in die Lage zu versetzen, sich trotz des
vorherrschenden Datenchaos ein praxistaugliches eigenes Urteil zu bilden.

Anzeige
Immer mehr im Gesundheitsssektor
engagierte Unternehmen haben die Marktlücke "Kater nach Alkoholexzess" entdeckt und vermarkten Produkte, die angeblich den Kater verhindern sollen.
Am bekanntesten ist das Produkt Myrkl des schwedischen Probiotika-Herstellers
De Faire Medical.
Myrkl ist ein
probiotisches
Nahrungsergänzungsmittel, das die
generische Wirksubstanz
AB001
enthält, die nach Angabe des Herstellers dafür sorgt, dass der im Blut
gelöste Alkohol bereits im Darm durch Aufspaltung
in seine Bestandteile "entschärft" wird - also bevor er die sensiblen Leberzellen erreichen und schädigen kann - und bevor die
natürlichen Spaltprodukte einen mit
Kopfschmerzen und Übelkeit
bzw. Sodbrennen verbundenen "Alkohol-Kater"
auslösen können.

Anzeige
Die angeblich weite Verbreitung von
Penicillin-Allergien, behindert die medikamentöse Behandlung von bakteriell bedingten Infektionen.
Die vom Patienten erinnerte Diagnose wird
selten überprüft und ist oft (bis
zu 90%?) falsch.
Sie führt zum unnötigen Einsatz
von teuren Reserve-Antibiotika und
fördert die Entwicklung weiterer Antibiotika-Resistenzen.
Preisgünstiges Penicillin könnte
nach erfolgtem Test, ohne Nebenwirkungen befürchten zu müssen, verordnet werden. Die
häufige Fehldiagnose
"Penicillin-Allergie"
führt zum unnötigen,
kontraproduktiven Einsatz teurer Reserve-Antibiotika. Auf der Website
www.allergietherapie.de/penicillinallergie
können sich Betroffene weiter informieren.
|
|
|
|

 zum Seitenanfang
zum Seitenanfang
|

|
Hier können Kooperationspartner die Mitglieder ihrer jeweiligen
Zielgruppen über ihr Unternehmen, ihre besonderen
Kompetenzgebiete, sowie die von ihnen angebotenen Produkte und
Dienstleistungen werbend informieren.
07.12.2021

Ehrenamtlich tätige Idealisten helfen mit Hilfe der kostenlosen Smartphone-App
Be My Eyes blinden und sehbehinderten Menschen
-weltweit und innerhalb weniger Sekunden und in über
hundert Sprachen- die Tücken des Alltags besser zu
meistern.
App-Download und Anmeldung
 Atemgas-Analysen
Atemgas-Analysen unterstützen Diagnostik und das Therapie-Management
wichtiger Krankheiten. Die Messdaten sind
von Ärzten und betroffenen Patienten schnell, kostengünstig
und nebenwirkungsfrei zu erheben.
Specialmed hat sich auf Atemgas-Analysen spezialisiert und bietet innovative
Atemgas-Analyse-
Geräte an. Schwerpunkte sind der
FeNO-Atemtest zur
Asthma-Diagnose und der
H2 Atemtest zur Diagnose einer
Laktose-Unverträglichkeit sowie der
CO-Atemtest zur Unterstützung der
Rauchenentwöhnung. Und
hier gehts zum
Specialmed-Shop
 Algen - Vielfalt aus dem Meer
Algen - Vielfalt aus dem Meer
Speisealgen sind in der asiatischen Küche weit verbreitet
und in Europa in erster Linie als Bestandteil von Sushi bekannt.
Man unterscheidet zwischen Mikro- und Makroalgen.Mikroalgen werden als
Nahrungsergänzungsmittel
angeboten - jetzt auch in Bio-Qualität. Die bekanntesten Mikroalgen sind
Spirulina,
Chlorella,
Astaxanthin und
AFA
.

 Gesunde
Kinder:
Gesunde
Kinder: Was viele
Frauen nicht wissen - ein Mangel am
Vitamin Folsäure
sollte schon vor Beginn einer
Schwangerschaft durch die Einnahme von
Folsäure-Tabletten ausgeglichen
werden.
mehr lesen
Dieses Medikament heisst Folarell und jede Frau kann es in der Apotheke ihres
Vertrauens rezeptfrei als "Pille zur Anti-Baby-Pille" zu kaufen.
Oder bequem hier in der online Apotheke
bestellen.
 Bio-Nutzhanf-Produkte:
Bio-Nutzhanf-Produkte:
Liebhaber unverfälschter Naturprodukte wollen
die Kräfte der Natur nutzen.
Innovative deutsche und
österreichische Unternehmen stellen
in enger Zusammenarbeit mit
engagierten Bio-Landwirten Nutzhanf-Produkte her,
die ausschließlich aus legalem,
EU-zertifiziertem, Saatgut gezogen
werden. Sie enthalten
Cannabidiol (CBD)und Cannabigerol
(CBG).
 Sanorell Pharma
Sanorell Pharma
empfiehlt das Fachbuch:
 Homöopunktur
Homöopunktur
Praktische Quantenmedizin.
Dr. med. Bernd Krautheimer. Die durch die Kombination von
Homöopathie
und Akupunktur erzielten Synergieeffekte
ermöglichen ein optimales ganzheitliches Therapie-Ergebnis.


Der
Sanorell
Vital-Test
hat ergeben, dass 47% der Frauen und 45% der
Männer nicht ausreichend mit
Vitaminen
und Spurenelementen versorgt sind.
Der Sanorell-Vital-Test zeigt, ob es
sinnvoll ist, einen entdeckten
Mangel
durch passende,
in jeder Apotheke rezeptfrei zu kaufende
Nahrungsergänzungsmittel auszugleichen. Über die
Versandapotheke
Fixmedika können Sie sich die
Vital Plus-Kombination kostengünstig zusenden lassen.
 Thymustherapie: Furcht vor dem Aus ist unbegründet.
Thymustherapie: Furcht vor dem Aus ist unbegründet.

Deutsche Verwaltungsgerichte
haben den Weg freigehalten, so dass die seit vielen
Jahren beliebte
Thymustherapie
auch in Zukunft auf dem Wege der Eigenherstellung der
Thymus-Peptide legal durchgeführt werden kann.
Das Unternehmen
Sanorell Pharma
verfügt
über alle behördlich vorgeschriebenen Genehmigungen und
unterstützt die an der Thymustherapie interessierten
Therapeutinnen und Therapeuten aktiv bei der Herstellung der
Thymus-Medikamente.
|
|